|
|
|
|
|
|
|
|
EpiGingival Tissue Model
(The EpiOral™ Tissue Model 바로가기)
Features
EpiGingival Tissue Model
- Gingival Phenotypes
- Multilayered Tissue like EpiOral, but with Cornified Apical Layers
- In Vivo-Like Lipid Profile
- Tissues Produce Human Beta Defensins (Antimicrobial Peptides)
- Ideal for Irritation, Oral Pathology and Basic Studies
General MatTek Tissue Features
- Unsurpassed Long-Term Tissue Reproducibility
- 3-Dimensional, Highly Differentiated Tissues
- Metabolically, Mitotically Active Tissues
- Produced from Normal (Non-Transformed) Human Cells - Ideal for Genomics Studies
- Produced in Easily Handled Cell Culture Inserts
- Grown in Completely Serum-Free Media System
- Quantifiable, Objective Test Endpoints
- Cost Effective Alternative to Animal and Human Clinical Testing
- List of Contract Testing Labs Qualified to Run MatTek Tissue-Based Tests Available

Applications
EpiGingivalTM Tissue Model
Irritation
Drug Delivery
Inflammation
Data Sheet
MatTek's EpiGingival tissues consist of normal, human-derived epithelial cells. The cells have been cultured to form multilayered, highly differentiated models of the gingival (EpiGingival) phenotypes.
The tissues, which are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro cell culture technology. Morphologically, the tissue models closely parallel native human tissues, thus providing a useful in vitro means to assess irritancy, disease and other basic oral biology phenomena.
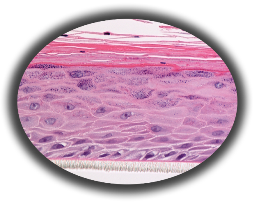
Figure 1: HISTOLOGY EpiGingival™ (gingival phenotype) Formalin Fixed, Paraffin Embedded, H&E Stained
The EpiGingival tissue model exhibits in vivo-like morphological and growth characteristics which are uniform and highly reproducible.
The EpiGingival tissue is also multilayered except that the apical layers are cornified, similar to in vivo gingival tissue (Figure 1).
The tissue expresses cytokeratin K13 in the all layers except the most apical ones and weakly express cytokeratin K14 in the upper layers of the tissue (Figure 2).
The tissues also produce the naturally occurring antimicrobial peptides called human beta defensins (HBD). As shown in Figure 3, the ORL-200 constitutively expresses HBD-1 and HBD-3 but not HBD-2. The GIN-100 tissue weakly expresses HBD-3 in all layers except the stratum corneum, expresses HBD-1 weakly only in the apical layers, and does not express HBD-2 (Figure 3).
Lipid analysis of the tissues revealed the presence of Ceramides 1, 2, and 3 (also referred to as Ceramides EOS, NS, and EOHP/NP, respectively) in the EpiGingival tissue, overlapping normal lipid profiles of in vivo tissue.
Various industrial and toxicology laboratories are actively seeking alternatives to expensive clinical or whole animal testing. Oral care, personal care and pharmaceutical companies have initiated in vitro toxicology testing to evaluate their raw materials and final product formulations.
A growing body of data indicates that EpiGingival effectively provides an inexpensive, non-animal means to assess gingival irritation, toxicology, and pathology related issues.
The protocols for using EpiGingival are clear and straightforward. The tissues have been utilized with several common tests of cytotoxicity and irritancy, including MTT and IL-1alpha.
mRNA can easily be harvested to analyze gene expression and the culture medium can be analyzed using ELISA assays to measure cytokine release.
Technicians find the rigid substrate design of EpiGingival easy to handle and manipulate in routine repetitive testing environments and scientists find that they are able to perform discriminating tests due to low background interference.

Figure 2: Immuno-staining of EpiGingival tissue for cytokeratin K13 and K14.
Figure 3: Immuno-staining of the reconstructed GIN-100 tissue for: A) human defensin beta (HBD)-1, B) HBD-2, and C) HBD-3. The negative control slide without the primary antibody is identical to hBD-2 slide. Objective = 20X.
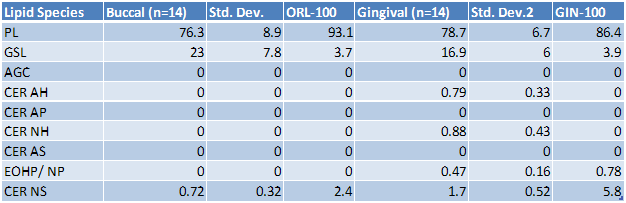
Table 1: Lipid analysis of in vivo and in vitro tissues. The in vivo buccal and gingival values are averages for 14 tissues samples. The in vitro tissue values are from a single lot.
Key: PL = Phospholipid, GSL = Glucosphingolipid, AGC = Acyl glucosylated ceramide, CER AH = ceramide 7, CER AP = ceramide 6, CER NH = ceramide 5, CER AS = ceramide 4, CER EOHP/NP = ceramide 3, CER NS = ceramide 2, CER EOS = ceramide 1 (1)
(1) Ponec M, Weerheim A, Lankhorst P, Wertz PW: New acylceramide in native and reconstructed epidermis. J Invest Dermatol 120:581-588, 2003.

Table 2: Effect of sodium dodecyl sulfate (SDS) on GIN-100 tissue. The tissue viability was determined using the MTT assay and the exposure time that reduces the tissue viability to 50% (ET-50) for each SDS solution was determined as follows:
GIN-100: 100 µL of neat solution applied.
Technical Specifications
I. Ordering EpiGingival
- New Orders: MatTek Scientists will consult on your application at no additional charge. It is STRONGLY RECOMMENDED that you have this consultation BEFORE placing your first order to help ensure that all media and accessories needed to perform your application are included in the initial order.
- ALL Tissue Orders: Please allow 3-4 weeks from order date to delivery of tissues. Please contact MatTek Customer Service for additional details.
II. Cells
- Type: Normal human oral keratinocytes (NHOK) are differentiated into tissues with a cornified, gingival phenotype, part number GIN-100.
- Genetic make-up: Single donor.
- Derived from: Non-diseased, adult human oral tissue obtained from patients undergoing tooth extractions.
- Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma, bacteria, yeast, fungi.
III. Medium
- Base medium: Dulbecco's Modified Eagle's Medium (DMEM).
- Growth factors/hormones: Epidermal growth factor and other proprietary factors.
- Serum: None.
- Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
- Anti-fungal agent: Amphotericin B 0.25 µg/ml.
- pH Indicator: Phenol red.
- Other additives: Proprietary.
- Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free medium and tissue are available. Agents are removed 3 days prior to shipment.
- Maintenance medium: GIN-100-MM.
IV. Tissue
- Kits: The EpiGingival kits (GIN-100) consist of 24 tissues. GIN-606 kits consist of 6 tissues. (Tissue "kits" contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
- Substrate: GIN-100: Collagen coated, 9 mm ID single well tissue culture plate inserts are used - Millipore Millicell™ CM single well tissue culture plate inserts. Pore size = 0.4 µm, Inner Diameter = 0.875 cm. Surface area = 0.6 cm2.
GIN-606: Millipore Millicell™ CM single well tissue culture plate inserts. Pore size = 0.4 µm, Inner Diameter = 2.5 cm. Surface area = 4.2 cm2.
- Culture: At air liquid interface.
- Histology: GIN-100: 8-11 cell layers of cornified tissue.
- Lot Numbers: Tissue lots produced by each technician for each week are assigned a specific lot number. Typically, there are multiple lot numbers for any given week's tissue production. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical with regard to cells, medium, handling, culture conditions, etc.
- Shipment: At 4°C on medium-supplemented, agarose gels.
- Shipment day: Monday.
- Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
- Shelf life: Including time in transit, tissues may be stored at 4°C for up to 4 days prior to use. However, extended storage periods are not recommended unless absolutely necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Wednesday morning following overnight storage at 4°C.
- Length of experiments: Cultures can be continued for at least 1 week with good retention of normal morphology. Cultures must be fed every other day with 5.0 ml of maintenance medium (GIN-100-MM). Cell culture inserts are placed atop culture stands (MEL-STND) or washers (EPI-WSHR) in 6-well plates to allow the use of 5.0 ml.
- Alternative tissues:
ORL-100-IB: Similar to ORL-200 except tissue is 20-30 cell layers thick.
ORL-606: Similar to ORL-200 except 6 tissues per kit, each tissue cultured in a larger diameter tissue culture insert.
ORL-300-FT: Full thickness ORL-200.
ORL-300-FT-LC: Full thickness ORL-200 with Langerhans Cells.

|
|
MEL-STND Accessory For Extended Culture Periods: Shown: 6-well plate with cell culture insert atop a culture stand (Part #MEL-STND). For experiments extending beyond 24 hours, tissues need to be fed with 5.0 ml every other day. Use of the culture stand allows feeding with 5.0 ml from the basolateral side of the tissue. Use of culture stands is necessary in order to maintain normal tissue morphology.
|
V. Quality Control & Sterility
- Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
- End-use testing: Transepithelial electrical resistance (TEER) of each lot is measured prior to shipment (QC specification: GIN-100: 516 + 122 ohms*cm2). Tissues are also exposed to 1.0% Triton X-100 for 60, 120 and 240 minutes. The time of exposure required to reduce the tissue viability (ET-50) using the MTT assay is determined (QC specification: GIN-100: 7.97 + 0.8 hrs.). A summary of these results will be provided to the customer for reference. (For TEER measurements, MatTek has successfully used the WPI Endohm System.)
- Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
- Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
- Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because some of our QC and sterility testing is done post-shipment, notification will be made as soon as possible. (Under normal circumstances, ET-50 failures will be notified by Thursday, 5 p.m.; sterility failures will be notified within 8 days of shipment.)











www.MatTek.co.kr
|
|
|
|
|