In Vitro Human Alveolar Tissue Model
for Inhaled Drug Delivery Applications
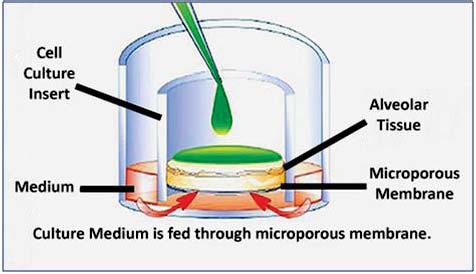
Figure 1: Schematic illustration of air/liquid interface (ALI) culture on microporous
membrane inserts. Because the apical tissue surface is not submerged in culture medium,
test articles and other exogenous stimuli (e.g.chemicals, virus, bacteria, aerosoles, etc.) can
be topically applied, as occurs in vivo.

Figure 2: Characterization of Passage 3 human alveolar type II epithelial cells in submerged monolayer culture. (A) Phase contrast photomicrograph (40X). (B) ICC staining for surfactant protein C marker for ATII cells (40X) (1).

Figure 3 : H&E stained paraffin section of human alveolar/endothelial co-culture tissue
differentiated at the ALI (40X).

Figure 4: Z-Stack confocal image of differentiated human alveolar/endothelial co-culture showing alveolar type I epithelial cytokeratin 19 staining (green) and endothelial von Willebrand factor staining (red).

Figure 5: Confocal staining of epithelial tight junction protein occludin in differentiated
epithelial/endothelial co-culture tissue (60X).

Figure 6: Confocal staining of endothelial tight junction protein e-cadherin in differentiated
epithelial/endothelial co-culture tissue (60X).

Figure 7: Timecourse of barrier development as determined by measurement of transepithelial
electrical resistance (TEER) and electrical potential difference (PD).

Figure 8: Confocal staining of alveolar type I epithelial cell carboxypeptidase M in
differentiated epithelial/endothelial coculture tissue (3).

Figure 9: RT-PCR of drug transporters and alveolar cell markers in differentiated epithelial/endothelial co-culture tissue.

Figure 10: Confocal imaging of human macrophages incorporated into differentiated
epithelial/endothelial co-culture tissue. CellTracker dye was used to visualize macrophages on the luminal side of the alveolar cultures.

포스터 요청은 이곳에서 해주세요.