|
|
|
|
|
|
|
|
The MelanoDerm TM Skin Model
주문은 최소 1개월전에 하셔야합니다.
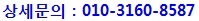
MelanoDerm-Specific Features:
- Co-Culture of Human Keratinocytes and Melanocytes
- Human 3-D Skin-Like Tissue Structure
- Black, Caucasian or Asian Melanocytes Available
- Progressive, Macroscopic Darkening
- Ideal for Skin Lightening/Whitening Studies, Melanogenesis Research
General MatTek Tissue Features:
- Unsurpassed Long-Term Tissue Reproducibility -
Lot-to-Lot, Year-to-Year
- 3-Dimensional, Highly Differentiated Tissues
- Metabolically, Mitotically Active Tissues
- Produced from Normal (Non-Transformed) Human Cells - Ideal for Genomics Studies
- Produced in Easily Handled Cell Culture Inserts
- Grown in Completely Serum-Free Media System
- Quantifiable, Objective Test Endpoints
- Cost Effective Alternative to Animal and Human Clinical Testing
- List of Contract Testing Labs Qualified to Run MatTek Tissue-Based Tests Available
Applications
MelanoDerm Tissue Model
- Skin Lightening
- Pigmentation Studies
Data Sheet
MatTek's MelanoDerm™ System consists of normal, human-derived epidermal keratinocytes (NHEK) and melanocytes (NHM) which have been cultured to form a multilayered, highly differentiated model of the human epidermis. The NHM within co-cultures undergo spontaneous melanogenesis leading to tissues of varying levels of pigmentation. The tissues are produced using serum free medium without artificial stimulators of melanogenesis such as TPA and IBMX. The cultures are grown on cell culture inserts at the air-liquid interface, allowing for topical application of skin lighteners or self-tanning agents. Thus, the model provides a useful in vitro means to evaluate cosmetic and pharmaceutical agents designed to modulate skin pigmentation.
The MelanoDerm Skin Model exhibits in vivo-like morphological and ultrastructural characteristics which are uniform and highly reproducible. NHM localized in the basal cell layer of MelanoDerm are dendritic and spontaneously produce melanin granules which progressively populate the layers of tissue. When cultured for up to 3 weeks (post-shipment), cultures become increasingly pigmented with retention of normal epithelial morphology. Cultures containing NHM derived from black donors show increased pigmentation versus those containing Caucasian-derived or Asian-derived NHM; all three types of cultures are distinctly darker than NHM-free cultures (EpiDerm™). The topical application of known inhibitors of melanogenesis significantly reduce melanin production and macroscopic darkening. Conversely, NHM within the tissue will respond to known stimulants of melanogenesis, such as -melanocyte stimulating hormone and -fibroblast growth factor, to produce tissues which darken faster than untreated controls. Various cosmetic and pharmaceutical laboratories are actively seeking alternatives to expensive and time consuming clinical and whole animal testing. Many companies have initiated MelanoDerm testing to assess the ability of their raw materials and final product formulations to modulate skin pigmentation. A growing body of data demonstrates that MelanoDerm provides an inexpensive, effective means of assessing various skin pigmentation issues while avoiding species extrapolation and the use of laboratory animals.
The protocols for using the MelanoDerm System are clear and straightforward. The organotypic cultures allow for topical or subcutaneous application of melanogenesis inhibitors or stimulators. Analytical methods have been developed to evaluate melanocyte dendricity and viability, pigment granule transfer to adjacent keratinocytes, bulk darkening of tissue, and total melanin content and synthesis rates. Finally, technicians find MelanoDerm's rigid substrate design easy to handle and manipulate.
H&E Stained parrafin section of MEL-300-B cultured for 14 days showing melanin granules (400X)
 |
|
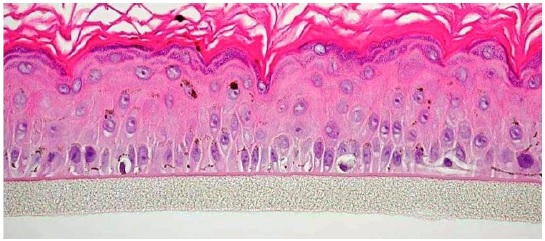
Top view of dendritic melanocytes within MelanoDerm (MEL-300-B) after 7 days of culture in Long Life Maintenance Medium (EPI-100-LLMM). Final magnification = 360X.
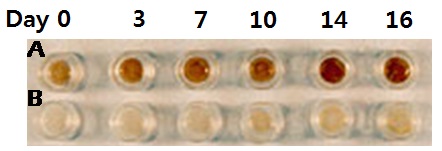
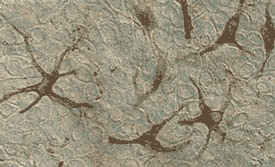
Comparison of macroscopic darkening in MelanoDerm tissue (MEL-300) containing melanocytes harvested from A) black and B) Caucasian donors.
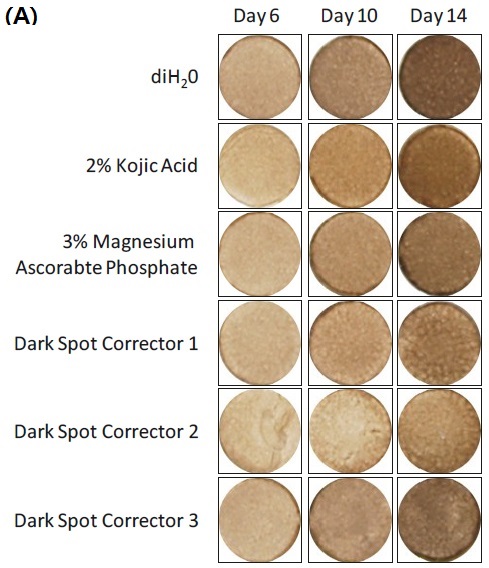
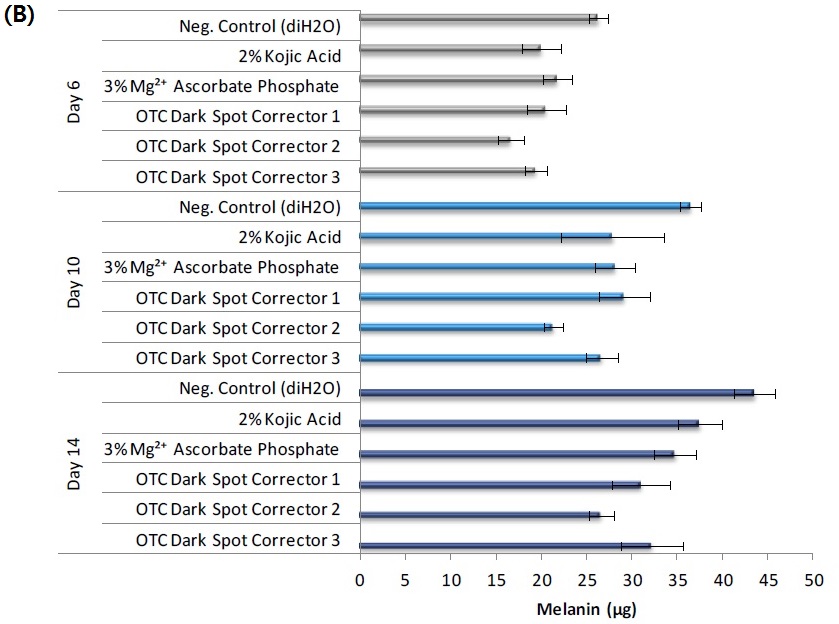
Effect of raw ingredient and final formulations on A) macroscopic darkening and B) melanin production in MelanoDerm (MEL-300-B). 25 µl of test material was applied to the apical surface of the tissue every 48 hours for a maximum of 14 days. Macroscopic darkening and melanin production were analyzed at days 6, 10 and 14.
|
 |
|
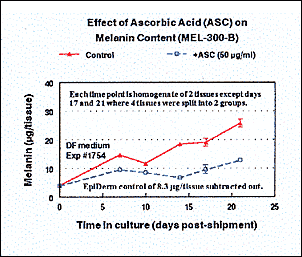
Melanin assay results for MEL-300-B tissue treated with ascorbic acid (50 ug/ml).
|
 |
|
Unstained histological cross-section of MEL-300-B tissue cultured in -LLMM for 7 days showing melanin granule population of tissue. Final Magnification = 360X. |
I. Ordering
- New Orders: MatTek Scientists will consult on your application at no additional charge. It is STRONGLY RECOMMENDED that you have this consultation BEFORE placing your first order to help ensure that all media and accessories needed to perform your application are included in the initial order.
- ALL Tissue Orders: Please allow 3-4 weeks from order date to delivery of tissues.
상담실로 바로가기
II. Cells
- Types: a) Normal human epidermal keratinocytes (NHEK); b) Normal human melanocytes (NHM).
- Genetic make-up: NHEK and NHM are from individual, but different donors. Tissue containing matched NHEK and NHM are available upon special request.
- Ethnic origin: MelanoDerm containing NHM from Caucasian (MEL-300-C), Black (MEL-300-B), and Asian (MEL-300-A) donors are available.
- Derived from: Neonatal-foreskin tissue.
- Alternatives: A limited number of strains of NHEK & NHM from adult breast tissue.
- Screened for: HIV, Hepatitis B, and Hepatitis C.
III. Medium
- Base medium: Dulbecco's Modified Eagle's Medium (DMEM).
- Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone, and other proprietary stimulators of epidermal differentiation.
- Serum: None.
- Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
- Anti-fungal agent: Amphotericin B 0.25 µg/ml.
- pH Indicator: Phenol red.
- Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary).
- Alternatives: Phenol red-free (MEL-300-B-PRF), antibiotic-free (MEL-300-B-ABF), anti-fungal-free (MEL-300-B-AFF), and hydrocortisone-free medium and tissue (MEL-300-B-HCF) are available. Agents are removed at least 3 days prior to shipment.
- Maintenance medium: Four maintenance media are available.
For long term experiments in which pigmentation and histology are important, the use of EPI-100-NMM, EPI-100-NMM-113, or EPI-100-LLMM is recommended.
EPI-100-NMM (New Maintenance Medium) contains KGF which preserves histology, but does not contain any factors that directly promote pigmentation.
EPI-100-NMM-113 contains KGF, β-FGF, and α-MSH and promotes pigmentation as well as retention of histology.
Long life maintenance medium, EPI-100-LLMM, contains α-MSH and β-FGF, but not KGF. Tissues will darken most rapidly in LLMM, less rapidly in NMM-113, and least rapidly in NMM.
The best retention of histology will be obtained when NMM or NMM-113 is used, and the cultures are fed 5.0 ml of media every 48 hr.
The 4th medium, EPI-100-MM, is a basal medium and that can be used for short term toxicological testing. It does not contain any factors that promote pigmentation. EPI-100-MM should not be used for experiments in which tissue histology is an important endpoint.
IV. Tissue
- Kit: Standard MelanoDerm™ kit (MEL-300) consists of 24 individual tissues, each tissue 9 mm in diameter. (Tissue "kits" contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
- Substrate: Chemically modified, collagen-coated, 9 mm ID single well tissue culture plate inserts are used (e.g. Millicell CM or Nunc polycarbonate single well tissue culture plate inserts).
- Culture: At air liquid interface.
- Seeding ratio: NHM:NHEK = 1/10. Ratio of 1/30 available upon request.
- Histology: 8-12 cell layers (basal, spinous, and granular layers).
- Stratum Corneum: 10-15 layers (based on TEM).
- Lot Numbers: Tissue lots produced by each technician for each week are assigned a specific lot number. Typically, there are multiple lot numbers for any given week's tissue production. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
- Shipment: At 4°C on medium-supplemented, agarose gels in 24-well plate.
- Shipment day: Every Monday. Shipment on Thursday also available upon special request.
- Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Wednesday depending on location.
- Shelf life: Including time in transit, tissues may be stored at 4°C for up to 6 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
- Length of experiments: Cultures can be continued for at least 2 weeks with good retention of normal epidermal morphology. Cultures must be fed every other day with 5.0 ml of either New Maintenance Medium (NMM) or NMM-113. Cell culture inserts are placed atop culture stands (MEL-STND) or washers (EPI-WSHR) in 6-well plates to allow use of 5.0 ml.
- Alternative tissues:
MEL-300-EACH: Same as MEL-300 except only 1 tissue, not 24 tissues.
MEL-300-B-FRZN-EA: MEL-300-B tissue that has been subjected to 3 freeze/thaw cycles. Designed to serve as control for melanin assay.
MEL-301: Cultured 3 days less than MEL-300, less differentiated than MEL-300
MEL-312 (-A, -B, -C): Identical to MEL-300 (-A, -B, -C) except 12 tissues per kit instead of 24.
MEL-606 (-A, -B, -C): Same as MEL-300 (-A, -B, -C) except 6 tissues per kit, each tissue cultured in a larger diameter cell culture insert (ID = 22 mm).
MEL-300-FT (-A, -B, -C): Full-thickness human skin tissue model composed of fibroblast-containing collagen matrix (dermis); normal human epidermal keratinocytes and normal human melanocytes (epidermis). 24 tissues per kit.
 |
|
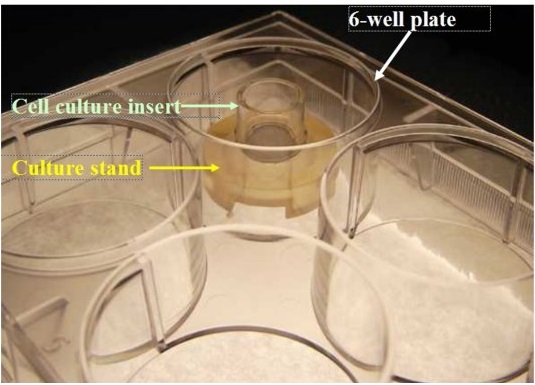
MEL-STND Accessory For Extended Culture Periods: Shown: 6-well plate with cell culture insert atop a culture stand (Part #MEL-STND). For experiments extending beyond 24 hours, tissues need to be fed with 5.0 ml every other day. Use of the culture stand allows feeding with 5.0 ml from the basolateral side of the tissue. Use of culture stands is necessary in order to maintain normal tissue morphology.
|
V. Quality Control and Sterility
- Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment. Select tissues are examined microscopically to insure that melanocytes are visible (for MEL-300-B and MEL-300-A tissues) and that they are evenly distributed throughout the tissue.
- End-use testing: Tissues are continued in EPI-100-LLMM to insure that macroscopic darkening of tissues occurs. Tissues are exposed to 1% Triton X-100 for 5 hrs. The viability at 5 hrs must exceed 45% as determined by the MTT assay (See MatTek EpiDerm™ ET-50 protocol) for each lot of tissue.
- Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
- Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
- Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, % viability failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment).




www.MatTek.co.kr
|
|
|
|
|