|
EpiAirway™ Applications
주문은 최소 1개월전에 하셔야 합니다.
EpiAirwayTM Tissue Model
- Respiratory Drug Delivery
- Respiratory Infection
- Respiratory Toxicology
- Nanoparticle Research
- Drug Discovery
- Drug Development
- Basic Respiatory Research
Disease Models
- Asthma
- Smoker
- COPD
- Goblet Cell Hyperplasia
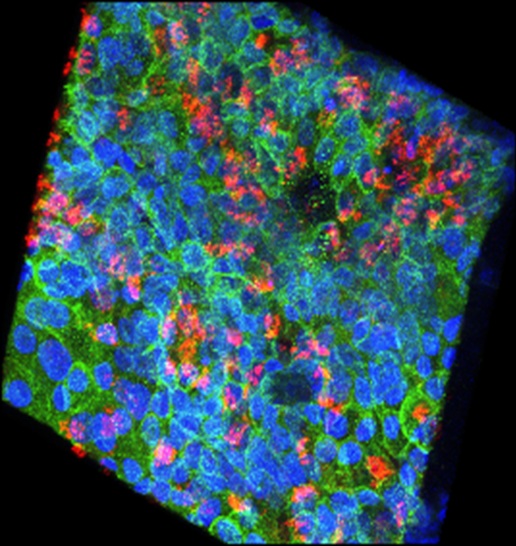
3D Confocal Image of EpiAirwayTM
- tight-junction protein (e-cadherin, green)
- cilia protein (α-tubulin, red)
- nuclei stain (DAPI, blue)
Overview
MatTek's EpiAirway tissues consist of normal, human-derived tracheal/bronchial epithelial (NHBE or TBE) cells which have been cultured to form a pseudo-stratified, highly differentiated model which closely resembles the epithelial tissue of the respiratory tract. Histological cross-sections of both the in vitro tissue and a normal human bronchiole reveal a pseudo-stratified mucociliary phenotype and transmission electron microscopy shows numerous microvilli and cilia on the apical surface of the cultures and confirmed the presence of tight junctions. For more information on the EpiAirway tissue model, click here.
Major Applications:
1. Respiratory Infection
2. Inhalation Toxicology
3. Asthma
4. COPD
5. Smoker
6. Nanoparticle Toxicology/Penetration
7. Drug Delivery,
8. Inflammation
1. Respiratory Infection

713. INHIBITION OF HUMAN RESPIRATORY SYNCYTIAL VIRUS INFECTIVITY BY A DENDRIMERIC HEPARAN SULPHATE-BINDING PEPTIDE.
Donalisio1, M., Rusnati2, M., Cagno1, V., Civra1, A., Bugatti2, A., Giuliani3, A., Pirri3, G., Volante1, M., Papotti1, M., Landolfo4, S., Lembo1, D. 1Department of Clinical and Biological Sciences, University of Turin, S. Luigi Gonzaga Medical School, Orbassano, Turin, Italy; 2Department of Biomedical Sciences and Biotechnology, University of Brescia, Italy; 3Spider Biotech s.r.l., Turin, Italy; 4Department of Public Health and Microbiology, University of Turin, Italy. Antimicrob. Agents Chemother., online ahead of print (July 2012).
711. CHARACTERIZATION OF EXTENDED CO-CULTURE OF NON-TYPEABLE HAEMOPHILUS INFLUENZAE WITH PRIMARY HUMAN RESPIRATORY TISSUES.
Ren1, D., Nelson2, K.L., Uchakin1, P.N., Smith2, A.L., Gu3, X-X and Daines1, D.A. 1Division of Basic Medical Sciences, Mercer University School of Medicine, Macon, GA; 2Seattle Children’s Research Institute, Seattle, WA; 3National Institute on Deafness and Other Communication Disorders, Rockville, MD, USA. Exper. Biol. and Medicine, 237, 540-547 (2012).
692. HIGHER LEVEL OF REPLICATION EFFICIENCY OF 2009 (H1N1) PANDEMIC INFLUENZA VIRUS THAN THOSE OF SEASONAL AND AVIAN STRAINS: KINETICS FROM EPITHELIAL CELL CULTURE AND COMPUTATIONAL MODELING.
Mitchell1, H., Levin2, D., Forrest2, S., Beauchemin3, C.A.A., Tipper1, J., Knight1, J., Donart1, N., Layton1, R.C., Pyles1, J., Gao1, P., Harrod1, K.S., Perelson4,A.S., and Koster1, F. 1Infectious Disease Program, Lovelace Respiratory Research Institute, Albuquerque, New Mexico 87108; 2Department of Computer Science, University of New Mexico, Albuquerque, New Mexico 87131; 3Department of Physics, Ryerson University, Toronto, Ontario M5B 2K3, Canada; and 4Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, New Mexico 87545. Journal of Virology, 85, (2),1125–1135 (2011).
663. MECHANISMS OF GOBLET CELL HYPERPLASIA INDUCED BY SIMULATED VIRAL EXPOSURE OR TH2 CYTOKINES.
Bolmarcich1, J., Wilbert2, S., Wright2, C., Jackson1, G.R., Kenney2, T., Baker2, B. and Hayden1, P. 1MatTek Corporation, Ashland, MA, USA. 2Gilead Sciences, Seattle, WA, USA. Presented at ATS Meeting, Denver, CO (2011).
659. USE OF THE EPIAIRWAY MODEL FOR CHARACTERIZING LONG-TERM HOST-PATHOGEN INTERACTIONS.
Ren, D., Daines, D.A. Division of Basic Medical Sciences, Mercer University School of Medicine.
626. A RECOMBINANT SIALIDASE FUSION PROTEIN EFFECTIVELY INHIBITS HUMAN PARAINFLUENZA VIRAL INFECTION IN VITRO AND IN VIVO.
562. ESTABLISHMENT OF A 3D AIRWAY MODEL FOR RESPIRATORY INFECTION.
Published by Battelle Memorial Institute. (2010).
553. THE EFFICACY OF ECHINACEA IN A 3-D TISSUE MODEL OF HUMAN AIRWAY EPITHELIUM.
Sharma1, M., Schoop2, R., and Hudson1, J.B. 1Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada, 1A.Vogel Bioforce AG, Roggwil, Switzerland. Phytother. Res., DOI: 10.1002/ptr.3051, 2009.
552. DAS181, A SIALIDASE FUSION PROTEIN, PROTECTS HUMAN AIRWAY EPITHELIUM AGAINST INFLUENZA VIRUS INFECTION: AN IN VITRO PHARMACODYNAMIC ANALYSIS.
Triana-Baltzer1, G.B., Babizki1, M., Chan2, M.C.W., Wong3, A.C.N., Aschenbrenner1, L.M., Campbell1, E.R., Li1, Q-X., Chan2, R.W.Y., Peiris2, J.S.M., Nicholls3, J.M., and Fang1, F. 1NexBio, Inc., San Diego, CA, USA; 2Department of Microbiology, University of Hong Kong, Pok Fu Lam, Hong Kong SAR; 3Department of Pathology, University of Hong Kong, Pok Fu Lam, Hong Kong SAR. J Antimicrob Chemother, doi:10.1093/jac/dkp421, 2009.
516. HUMAN PARAINFLUENZA VIRUS INFECTION OF THE AIRWAY EPITHELIUM: THE VIRAL HEMAGGLUTININ-NEURAMINIDASE REGULATES FUSION PROTEIN ACTIVATION AND MODULATES INFECTIVITY.
Palermo1, L.M., Porotto1, M., Yokoyama1, C.C., Palmer1, S.G., Mungall1,2, B.A., Greengard1,4, O., Niewiesk3, S., Moscona1, A. 1Department of Pediatrics and of Microbiology and Immunology, Weill Medical College of Cornell University, New York, NY 10021. 2Australian Animal Health Laboratory, CSIRO Livestock Industries, 5 Portarlington Road, Geelong, Australia 3220. 3Department of Veterinary Biosciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210. 4Department of Pediatrics, Mount Sinai Hospital, NY. J. Virol., doi:10.1128/JVI.00475-09.
509. ROLE OF TOLL-LIKE RECEPTOR (TLR) ACTIVATION IN ASTHMA EXACERBATION: EXPERIMENTS WITH IN VITRO MODELS OF HUMAN AIRWAY EPITHELIAL CELLS (EPIAIRWAY) AND EPITHELIAL CELL/FIBROBLAST CO-CULTURES (EPIAIRWAY-FT).
Hayden1, P.J., Bolmarcich1, J., Armento1, A., Jackson, Jr.1, G.R., Hackett2, T.L., Knight2, D.A. and Klausner1, M. 1 MatTek Corporation, Ashland, MA. 2 James Hogg iCAPTURE Centre for Cardiovascular and Pulmonary Research, St. Paul's Hospital, Vancouver, BC, Canada. Presented at American Thoracic Society Annual Meeting (2009).
414. FORMULATION DEVELOPMENT OF siRNAs FOR DRUG DELIVERY: A NEW THERAPEUTIC APPROACH FOR PANDEMIC INFLUENZA.
Prieve, M.G., Ge, Q., Lamharzi, N., Cui, K., Thomson, A., Thompson, S., Templin, M., Wang, H., Farber, K., Roth, S., Houston, M., Johnson, P.H. Nastech Pharmaceutical Company, Inc., Bothell, Washington. Presented at the American Association of Pharmaceutical Sciences (AAPS) Biotechnology Conference, Boston, MA, June 18-21 (2006).
156. RECONSTRUCTED, DIFFERENTIATED AIRWAY EPITHELIAL CULTURES TO MODEL RESPIRATORY INFECTION.
Klausner1, M., Kubilus1, J., Ogle1, P., Adamou2, J.E., Langermann2, S. 1MatTek Corp., Ashland, MA 01721, 2MedImmune Incorporated, Gaithersburg, MD 20878. Presented at the Lovelace Respiratory Research Institute symposium, "Validity of animal models of human respiratory diseases", Santa Fe, NM, October 1, (1998).
2. Inhalation Toxicology
MTT Tissue Viability Assay

700. CHARACTERIZATION OF DIACETYL VAPOR-INDUCED AIRWAY TOXICITY USING THE HUMAN EPIAIRWAY™ IN VITRO MODEL.
Gwinn1, W.M., Flake1, G.P., Bousquet2, R.W. and Morgan1, D.L. 1Respiratory Toxicology, NTP Laboratories, NTP, NIEHS, NIH, DHHS, RTP, NC; 2Alion Science and Technology Corp., RTP, NC.
684.USE OF NORMAL HUMAN 3-DIMENSIONAL (NHU-3D) TISSUE MODELS (EPIDERM™, EPIAIRWAY™) FOR NANOTOXICOLOGY APPLICATIONS.
Hayden, P., Kaluzhny, Y., Armento, A., DeLuca, J., Karetsky, V., Kubilus, J., Ayehunie, S., Kandarova, H. and Klausner, M. MatTek Corporation, Ashland, MA, USA & MatTek In Vitro Life Science Laboratories, Bratislava, Slovakia. Presented at Genetic Toxicology Association Meeting, Newark, DE, USA, September 2011.
683. UPDATE ON VALIDATION STATUS AND INDUSTRY UTILIZATION OF NORMAL HUMAN 3D (NHU-3D) ANIMAL ALTERNATIVE MODELS.
Kandarova, H., Klausner, M., Kubilus, J., Ayehunie, S., Hayden, P., Kaluzhny, Y., Letasiova, S., and Sheasgreen, J. MatTek Corporation, Ashland, MA, USA and MatTek In Vitro Life Science Laboratories, Bratislava, Slovakia. Presented at 8th World Congress on Alternative and Animal Use, Montreal, Canada, 2011
628. SULFUR MUSTARD VAPOR EFFECTS ON DIFFERENTIATED HUMAN LUNG CELLS.
Seagrave, JC., Weber, W.M. and Grotendorst, G.R. Lovelace Respiratory Research Institute, Albuquerque, NM, USA Inhalation Toxicology, Early Online 1-7, (2010)
564. ASSAY DEVELOPMENT FOR IN VITRO EVALUATION OF INHALATION TOXICITY USING THE EPIAIRWAY ORGANOTYPIC IN VITRO HUMAN AIRWAY MODEL.
Jackson, Jr., G.R., Bolmarcich, J., Oldach, J., Klausner, M. and Hayden, P.J. MatTek Corporation, Ashland, Massachusetts, USA. Presented at 49th Annual SOT Meeting, Salt Lake City, Utah (2010) and IIVS In Vitro Alternatives 2010 Forum.
561. NON-ANIMAL APPROACHES FOR CONSUMER SAFETY RISK ASSESSMENTS: UNILEVER'S SCIENTIFIC RESEARCH PROGRAMME.
Carmichael, P., Davies, M., Dent, M., Fentem, J., Fletcher, S., Gilmour, N., MacKay, C., Maxwell, G., Merolla, L., Pease, C., Reynolds, F., and Westmoreland, C. Safety & Environmental Assurance Centre (SEAC), Unilever, Colworth Science Park, Sharnbrook, Bedford, UK. ATLA, 37, 595–610, (2009).
456. AN IN VITRO MODEL OF HUMAN AIRWAY EPITHELIUM (EPIAIRWAY) FOR IN VITRO METABOLISM, TOXICITY SCREENING AND DRUG DELIVERY APPLICATIONS.
Bolmarcich, J., Stolper, G., Jackson, G.R., Klausner, M., Hayden, P.J. MatTek Corporation, Ashland, MA. Presented at 6th World Congress on Alternatives and Animal Use, Tokyo, August (2007).
384. IN VITRO ALTERNATIVES IN INHALATION TOXICOLOGY - WHERE DO WE START?
Greenwell1, L., Roggen2, E., Holder3, J., Westmoreland1, C., Cathew1, P., Fentem1, J. 1Safety and Environment Assurance Centre, Unilever Colworth, Sharnbrook, Bedford, U.K., 2Novozyme A/S, Smoermoseve, Bagsvaerd, Denmark, 3Glaxo-Smith-Kline, Ware, Hertfordshire, U.K. Presented at the 5th World Congress, Berlin, Germany, August 21-25, (2005).
383. MODELLING THE HUMAN BRONCHI AND ITS RESPONSES IN VITRO.
Greenwell, L., Carthew, P., Westmoreland, C., and Fentem, J. Safety and Enviornmental Assurance Centre, Unilever Colworth, Sharnobrook, Bedford, U.K. Presented at 5th World Congress, Berlin, Germany, August, (2005).
347. THE USE OF FLUORESCENTLY LABELED NANOPARTICLES TO DETERMINE THE EFFECT OF PARTICLE SIZE ON TRANSLOCATION FROM THE LUNG.
Carter1, J.M., Kennedy1, J.M., Oberdorster2, G., Clark3, E.D. 1The Procter & Gamble Co., Cincinnati, OH; 2University of Rochester, Rochester, NY; 3The Health and Environmental Safety Alliance, Cincinnati, OH. Presented at Society of Toxicology Annual Meeting, Baltimore, MD. March 21-25, (2004). The Toxicologist, 78, S1, 440 (2004).
300. IN VITRO MODELS OF FULL-THICKNESS HUMAN SKIN (EPIDERMFT™) AND AIRWAY EPITHELIUM (EPIAIRWAYFT™) FOR TOXICOLOGY AND DRUG DEVELOPMENT APPLICATIONS.
P.J. Hayden, M. Klausner, J. Kubilus, B. Burnham and G.R. Jackson. MatTek Corporation, Ashland, MA 01721. Presented at the 2003 Congress on In Vitro Biology, Portland, OR (May 31-June 4).
3. Asthma
Technical References ---> 바로가기
4. COPD
Technical References ---> 바로가기
5. Smoker
Technical References ---> 바로가기
6. Nanoparticle Toxicology/Penetration
Technical References

714. ENHANCED ANTIVIRAL ACTIVITY OF ACYCLOVIR LOADED INTO NANOPARTICLES.
Cavalli1, R., Donalisio2, M., Bisazza1, A., Civra2, A., Ranucci3, E., Ferruti3, P. and Lembo2, D. 1Dipartimento di Scienza e Tecnologia del Farmaco, Universita` di Torino, Torino, Italy. 2Dipartimento di Scienze Cliniche e Biologiche, Universita` di Torino, Torino, Italy. 3Dipartimento di Chmica Organica e Industriale, Universita` degli Studi di Milano, Milano, Italy. Methods in Enzymology, 509, (2012).
684. USE OF NORMAL HUMAN 3D (NHU-3D) TISSUE MODELS (EPIDERM™, EPIAIRWAY™) FOR NANOTOXICOLOGY APPLICATIONS
Hayden, P., Kaluzhny, Y., Armento, A., DeLuca, J., Karetsky, V., Kubilus, J., Ayehunie, S., Kandarova, H. and Klausner, M. MatTek Corporation, Ashland, MA, USA & MatTek In Vitro Life Science Laboratories, Bratislava, Slovakia. Presented at Genetic Toxicology Association Meeting, Newark, DE, USA, September 2011.
387. PROTECTING THE SKIN AGAINST OZONE.
Gruber1, J.V., Tay1, A., Holtz2, R. 1Arch Personal Care Products, South Plainfield, NJ; 2BioInnovation Laboratories, Inc., McKinney, TX. Journal of Cosmetic Sciences, 56, (5), 348-349, (2005).
7. Drug Delivery
Technical References

731. APPLICATION OF ORGANOTYPIC IN VITRO HUMAN CELL CULTURE MODELS FOR RESEARCH AND DEVELOPMENT OF INHALATION PHARMACEUTICAL FORMULATIONS TARGETING THE PROXIMAL AIRWAYS.
Hayden, P. MatTek Corporation, Ashland, MA. Inhalation Magazine (2012).
594. EFFECT OF CYCLODEXTRINS ON THE COMPLEXATION AND NASAL PERMEATION OF MELATONIN.
Babu1, R.J., Dayal2, P.,and Singh2, M. 1Department of Pharmaceutical Sciences, Harrison School of Pharmacy, Auburn University, Auburn, Alabama, USA. 2College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, Florida, USA. Drug Delivery, 15, 381-388, 2008.
592. IDENTIFICATION OF TIGHT JUNCTION MODULATING LIPIDS.
Chen-Quay, S-C., Eiting, K.T., Li, A.W.-A., Lamharzi, N., Quay, S.C. . Nastech Pharmaceutical Company, Inc., 3830 Monte Villa Parkway, Bothell, Washington. J. of Pharmaceutical Sciences, 98, 2, 2009.
578. PHARMACOLOGICAL PROPERTIES OF N-(3,5-DIAMINO-6-CHLOROPYRAZINE- 2-CARBONYL)-N’-4-[4-(2,3-DIHYDROXY-PROPOXY)PHENYL]BUTYL-GUANIDINE METHANE-SULFONATE (552-02), A NOVEL EPITHELIAL SODIUM CHANNEL BLOCKER WITH POTENTIAL CLINICAL EFFICACY FOR CYSTIC FIBROSIS.
Hirsh1, A.J., Zhang2, J., Zamurs1, A., Fleegle1, J., Thelin3, W.R., Caldwell3, R.A., Sabater4, J.R., Abraham4, W.M., Donowitz5, M., Cha5, B., Johnson1, K.B., St. George1, J.A., Johnson1, M.R., and Boucher3, R.C. 1Parion Sciences Inc., Durham, North Carolina; 2Albany Molecular Research Inc., Albany, New York; 3Cystic Fibrosis/Pulmonary Research and Treatment Center, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; 4Division of Pulmonary and Critical Care Medicine, University of Miami at Mount Sinai Medical Center, Miami Beach, Florida; and 5Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland. J. of Pharma. and Exper. Therapeutics, 325(1), 77-78, 2008.
572. AN IN VITRO HUMAN AIRWAY MODEL FOR RAPID TOXICITY AND FORMULATION SCREENING OF NASAL DRUG DELIVERY SYSTEMS.
Hayden, P.J., Bolmarcich, J., Jackson Jr., G.R., Cohen, H., Stolper, G., and Klausner, M. MatTek Corporation, Ashland, MA, USA. Presented at AAPS 2012.
553. THE EFFICACY OF ECHINACEA IN A 3-D TISSUE MODEL OF HUMAN AIRWAY EPITHELIUM.
Sharma1, M., Schoop2, R., and Hudson1, J.B. 1Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada, 1A.Vogel Bioforce AG, Roggwil, Switzerland. Phytother. Res., DOI: 10.1002/ptr.3051, 2009.
552. DAS181, A SIALIDASE FUSION PROTEIN, PROTECTS HUMAN AIRWAY EPITHELIUM AGAINST INFLUENZA VIRUS INFECTION: AN IN VITRO PHARMACODYNAMIC ANALYSIS.
Triana-Baltzer1, G.B., Babizki1, M., Chan2, M.C.W., Wong3, A.C.N., Aschenbrenner1, L.M., Campbell1, E.R., Li1, Q-X., Chan2, R.W.Y., Peiris2, J.S.M., Nicholls3, J.M., and Fang1, F. 1NexBio, Inc., San Diego, CA, USA; 2Department of Microbiology, University of Hong Kong, Pok Fu Lam, Hong Kong SAR; 3Department of Pathology, University of Hong Kong, Pok Fu Lam, Hong Kong SAR. J Antimicrob Chemother, doi:10.1093/jac/dkp421, 2009.
543. RETAINING CELL INTEGRITY DURING ORGANOTYPIC MODEL VIABILITY ASSAYS: ALTERNATIVES TO MTT.
Kavanagh1, C.R., Crawford1, L.J., Sawicka3, K.M., Simon1,2, S.R., and Roemer1,2, E.J. Departments of 1Pathology, 2Biochemistry and Cell Biology and 3BioMedical Engineering, SUNY Stony Brook, Stony Brook, NY, 11794. Presented at Society of In Vitro Biol. Meeting, Charleston, SC, June 2009.
542. VALIDATING MTS AS AN ALTERNATIVE VIABILITY ASSAY TO MTT ON THE HUMAN 3-D TISSUE MODELS, EPIAIRWAY AND EPIDERM.
Kavanagh1, C.R., Crawford1, L.J., Simon1,2, S.R. and Roemer1,2, E.J. Departments of 1Pathology, and 2Biochemistry and Cell Biology, SUNY Stony Brook, Stony Brook, NY, 11794. Presented at Society of In Vitro Biol. Meeting, Charleston, SC, June 2009.
508. IN VITRO FORMULATION OPTIMIZATION OF INTRANASAL GALANTAMINE LEADING TO ENHANCED BIOAVAILABILITY AND REDUCED EMETIC RESPONSE IN VIVO.
Leonard, A.K., Sileno, A.P., Brandt, G.C., Foerder, C.A., Quay, S.C., Costantino, H.R. Nastech Pharmaceutical Company Inc., 3450 Monte Villa Parkway, Bothell, WA 98021, USA. Intl. J. of Pharma., 335, 138-146, (2007).
495. RECONSTRUCTED EPIDERMIS AND FULL-THICKNESS SKIN FOR ABSORPTION TESTING: INFLUENCE OF THE VEHICLES USED ON STEROID PERMEATION.
Schäfer-Korting1, M., Mahmoud1, A., Borgia1, S.L., Brüggener1, B., Kleuser1, B., Schreiber1, S., and Mehnert2, W. 1Freie Universität Berlin, Institut für Pharmazie (Pharmakologie & Toxikologie), Berlin, Germany; 2Freie Universität Berlin, Institut für Pharmazie (Pharmazeutische Technologie), Berlin, Germany. ATLA, 36, 441-452, (2008).
456. AN IN VITRO MODEL OF HUMAN AIRWAY EPITHELIUM (EPIAIRWAY) FOR IN VITRO METABOLISM, TOXICITY SCREENING AND DRUG DELIVERY APPLICATIONS.
Bolmarcich, J., Stolper, G., Jackson, G.R., Klausner, M., Hayden, P.J. MatTek Corporation, Ashland, MA. Presented at 6th World Congress on Alternatives and Animal Use, Tokyo, August (2007).
448. IN VITRO AND IN VIVO SCREENING OF INTRANASAL INSULIN FORMULATIONS.
Cohen, A.S., Sileno, A.P., Peddakota, L.R., Forseth, K.T., Slater, A., Haugaard, D., Foerder, C.A., Brandt, G.C., Quay, S.C., and Costantino, H.R. Nastech Pharmaceuticals, Bothell, WA. Presented at the American Association of Pharmaceutical Scientists (AAPS) Biotechnology Conference, Boston, MA, June 18-21, (2006).
415. PEPTIDES AND PEGYLATED PEPTIDES AS INTRANASAL PERMEATION ENHANCERS: COMPARISON TO SMALL MOLECULAR EXCIPIENTS.
Lamharzi, N., Sileno, T., Eiting, K.T., Templin, M., Haugaard, K.D., Houston, M., Cui, K., Johnson, P.H., Chen, S-C. Nastech Pharmaceutical Company, Inc., Bothell, Washington. Journal of Pharmaceutical Sciences, 95, (6) 1364-1371 (2006).
414. FORMULATION DEVELOPMENT OF siRNAs FOR DRUG DELIVERY: A NEW THERAPEUTIC APPROACH FOR PANDEMIC INFLUENZA.
Prieve, M.G., Ge, Q., Lamharzi, N., Cui, K., Thomson, A., Thompson, S., Templin, M., Wang, H., Farber, K., Roth, S., Houston, M., Johnson, P.H. Nastech Pharmaceutical Company, Inc., Bothell, Washington. Presented at the American Association of Pharmaceutical Sciences (AAPS) Biotechnology Conference, Boston, MA, June 18-21 (2006).
413. THERAPEUTIC UTILITY OF A NOVEL TIGHT JUNCTION MODULATING PEPTIDE FOR ENHANCING INTRANASAL DRUG DELIVERY.
Chen, S.C., Eiting, K., Cui, K., Leonard, A.K., Morris, D., Li, C.Y., Farber, K., Sileno, A.P., Houston, M.E., Johnson, P.H., Quay, S.C., Costantino, H.R. Nastech Pharmaceutical Company, Inc., Bothell, Washington. Journal of Pharmaceutical Sciences, 95, (6) 1364-1371 (2006).
409. DRUG/XENOBIOTIC-METABOLIZING ENZYME (XME) EXPRESSION IN THE EPIAIRWAY IN VITRO HUMAN AIRWAY MODEL: UTILITY FOR ASSESSING TRACHEAL/BRONCHIAL BIOTRANSFORMATION OF INHALED PHARMACEUTICALS AND ENVIRONMENTAL CHEMICALS.
Hayden, P.J., Bolarcich, J., Stopler, G., Jackson, G.R., Klausner, M. MatTek Corporation, Ashland, MA, USA. Presented at the American Thoracic Society 2006 Annual Meeting, San Diego CA, May 19-24 (2006).
394. LONG TERM CULTURE OF PSEUDOSTRATIFIED TRACHEAL BRONCHIAL EPIAIRWAY™ TISSUES.
Hayden, P., Jackson, G.R. MatTek Corporation, Ashland, MA, United States. Internal results, (2006).
391. PEPTIDE DRUG PERMEATION ENHANCEMENT BY SELECT CLASSES OF LIPIDS.
Chen, S-C., Eiting, K.T., Li1, A.A.W., Lamharzi, N. and Quay, S.C. Nastech Pharmaceutical Company Inc. 3450 Monte Villa Parkway, Bothell, WA 98021, USA. 1Bioengineering, University of Washington. Presented at the 45th American Society of Cell Biology, San Francisco, CA, December 10-14, (2005).
390. DEVELOPMENT OF A NOVEL HIGH-CONCENTRATION GALANTAMINE FORMULATION SUITABLE FOR INTRANASAL DELIVERY.
Leonard, A.K., Sileno, A.P., MacEvilly, C., Foerder, C.A., Quay, S.C., and Costantino, H.R. Nastech Pharmaceutical Company Inc., 3450 Monte Villa Parkway, Bothell, WA 98021. Journal of Pharmaceutical Sciences, 94, 1736-1746, (2005).
368. INTRANASAL DELIVERY OF RECOMBINANT HUMAN PARATHYROID HORMONE [HPTH (1–34)], TERIPARATIDE IN RATS.
Agu1, R.U., Valiveti1, S., Earles1, D.C., Klausner2, M., Hayden2, P.J., Wermeling1,3,D.P., Stinchcomb1, A.L. 1College of Pharmacy, University of Kentucky, Lexington, Kentucky, USA; 2MatTek Corporation, Ashland, Massachusetts, USA; 3Intranasal Technology, Inc., Lexington, Kentucky, USA. Endocrine Research, 30, (3), 455-467, (2004).
345. LOCALIZATION OF P-GLYCOPROTEIN IN HYMAN TRACHEAL/BRONCHIAL EPITHELIAL CELL CULTURE (EPIAIRWAY™)
Chemuturi1, N., Hayden2, P., Klausner2, M., Donovan1, M. 1Division of Pharmaceutics, The University of Iowa, Iowa City, IA; 2MatTek Corporation, Ashland, MA. Presented at American Association of Pharmaceutical Scientists Annual Meeting, Baltimore, MD. November 7-10, (2004).
344. CANNABIDIOL DIFFUSION ACROSS HUMAN TRACHEO-BRONCHIAL TISSUE (EPIAIRWAY™) AND INTRANASAL ABSORPTION IN RATS.
Agu1, R., Valiveti1, S., Paudel1, K., and Klausner2, M., Hayden2, P. University of Kentucky College of Pharmacy, Lexington, KY; 2MatTek Corporation, Ashland, MA. Presented at American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, Baltimore, MD. November 7-10, (2004).
331. PERMEATION OF WIN 55,212-2, A POTENT CANNABINOID RECEPTOR AGONIST, ACROSS HUMAN TRACHEO-BRONCHIAL TISSUE (EPIAIRWAY™) IN VITRO AND RAT NASAL EPITHELIUM IN VIVO.
Agu1, R.U., Valiveti1, S., Paudel1, K.S., Klausner2, M., Hayden2, P.J., Stinchcomb1, A.S. 1University of Kentucky College of Pharmacy, Lexington, KY, 2MatTek Corporation, Ashland, MA. Journal of Pharmacy and Pharmacology, 58, 1-7, (2006).
315. DRUG PERMEABILITY ACROSS BOVINE RESPIRATORY TISSUES COMPARED TO A HUMAN TRACHEO/BRONCHIAL CELL CULTURE (EPIAIRWAY™ SYSTEM).
Chemuturi1, N., Donovan1, M., Klausner2, M., and Hayden2, P. 1College of Pharmacy, University of Iowa, Iowa City, IA. 2MatTek Corporation, Ashland, MA. Poster presented at the American Association of Pharmaceutical Scientists (AAPS), Salt Lake City, UT, October 26-30, (2003).
314. DOPAMINE TRANSPORT ACROSS RESPIRATORY EPITHELIUM.
Chemuturi1, N., Donovan1, M., Klausner2, M., and Hayden2, P. 1College of Pharmacy, University of Iowa, Iowa City, IA. 2MatTek Corporation, Ashland, MA. Poster presented at the American Association of Pharmaceutical Scientists (AAPS), Salt Lake City, UT, October 26-30, (2003).
310. DEVELOPMENT OF AN INTRANASAL FORMULATION OF THE Y2R AGONIST PEPTIDE YY3-36.
Kleppe, M., Deshpande, A., Go, Z., Constantino, H.R., Sileno, A., Brandt, G., Lee*, M., Quay, S. Nastech Pharmaceuticals, Bothell, WA and *UCLA, Los Angeles, CA.
271. AN IN VITRO MODEL FOR THE RAPID SCREENING OF POTENTIAL COMPONENTS AND FORMULATIONS FOR NASAL DRUG DELIVERY.
Scotto-Lavino, E., Easow, J., Simon, S., and Roemer, E., State University of New York (SUNY) at Stony Brook, Stony Brook, NY. In Vitro Cellular and Developmental Biology, 38, 12A (2002).
244. EFFECTS OF ENHANCERS ON MACROMOLECULES FORMULATIONS ON MEMBRANE PENETRATION, CELL VIABILITY AND RESISTANCE USING EPIAIRWAY™ TISSUE MODEL.
Quay, S.C., deMeireles, J., Wormuth, D., Vangalla, S., and Biswas, M. Nastech Pharmaceutical Company, Hauppauge, NY, USA. AAPSPharmSci, Vol. 3, No. 3, S-57, (2001).
243. PERMEABILITY AND CYTOTOXICITY OF MACROMOLECULE FROM NASAL FORMULATION USING EPIAIRWAY TISSUE MODEL.
El-Shafy, M.A., Roemer, E., deMeireles, J., Biswas, M., Quay, S.C. Nastech Pharmaceutical Company, Hauppauge, NY, USA. AAPSPharmSci, Vol. 3, No. 3, S-58, (2001).
8. Inflammation
Technical References

731. APPLICATION OF ORGANOTYPIC IN VITRO HUMAN CELL CULTURE MODELS FOR RESEARCH AND DEVELOPMENT OF INHALATION PHARMACEUTICAL FORMULATIONS TARGETING THE PROXIMAL AIRWAYS.
Hayden, P. MatTek Corporation, Ashland, MA. Inhalation Magazine (2012).
700. CHARACTERIZATION OF DIACETYL VAPOR-INDUCED AIRWAY TOXICITY USING THE HUMAN EPIAIRWAY™ IN VITRO MODEL.
Gwinn1, W.M., Flake1, G.P., Bousquet2, R.W. and Morgan1, D.L. 1Respiratory Toxicology, NTP Laboratories, NTP, NIEHS, NIH, DHHS, RTP, NC; 2Alion Science and Technology Corp., RTP, NC.
581. ASTHMATIC AIRWAY EPITHELIUM IS INTRINSICALLY INFLAMMATORY AND MITOTICALLY DYSSYNCHRONOUS.
Freishtat1,2,3, R.J., Watson3, A.M., Benton3, A.S., Iqbal1,2,3, S.F., Pillai1,3,4, D.K., Rose1,3, .M.C. and Hoffman1,3, E.P. 1Department of Integrative Systems Biology, George Washington University School of Medicine and Health Sciences; 2Division of Emergency Medicine, Department of Pediatrics, George Washington University School of Medicine and Health Sciences; 3Center for Genetic Medicine Research, Children’s National Medical Center; and 4Division of Pulmonary Medicine, Department of Pediatrics, George Washington University School of Medicine and Health Sciences, Washington, DC. Am J Resp Cell Mol Biol, 44,863-869, 2011
481. EXPOSURE OF A MULTI-CELLULAR IN VITRO MODEL OF HUMAN AIRWAY TISSUE TO WHOLE CIGARETTE SMOKE.
Walsh, P.T., Chalupowicz, D.G., Frankowski, R., Barnette, M. Discovery Biology, Respiratory CEDD, GlaxoSmithKline, Upper Merion, PA19406, USA. Amer. J. of Respiratory and Critical Care Medicine, Vol 177 (Abstracts Issue): pA728 (2008).
446. CYTOKINE-INDUCED GOBLET CELL HYPERPLASIA IN THE (EPIAIRWAY-FT) IN VITRO FULL-THICKNESS HUMAN AIRWAY MODEL.
Hayden, P.J., Jackson, G.R., Bolmarcich, J.L., Stolper, G., Spiller, E. and Klausner, M. MatTek Corporation, Ashland, MA, United States. Presented at the American Thoracic Society Annual Meeting, San Francisco, CA, May 22, Poster No. B33 (2007).
410. TH2 CYTOKINE STIMULATED AIRWAY EPITHELIAL CELLS DEMONSTRATE GRANULE-DEPENDENT FasL LOCALIZATION.
Singhera, G.K., Sekhon, M.S., Dorscheid, D.R. The James Hogg iCAPTURE Centre for Cardiovascular and Pulmonary Research, St. Paul’s Hospital / Providence Health Care-University of British Columbia, Vancouver, BC, Canada. Presented at the American Thoracic Society Annual Meeting, San Diego, CA., May 19-24 (2006).
394. LONG TERM CULTURE OF PSEUDOSTRATIFIED TRACHEAL BRONCHIAL EPIAIRWAY™ TISSUES.
Hayden, P., Jackson, G.R. MatTek Corporation, Ashland, MA, United States. Internal results, (2006).
370. MICROARRAY EXPRESSION STUDIES ON AN IN VITRO MODEL OF HUMAN AIRWAY EPITHELIUM (EPIAIRWAY).
Moffatt1, M.F., Hill1, A.L., Hao1, L., Taylor2, J., Hayden3, P.J., Cookson1, W.O.C.M. 1Wellcome Trust Centre for Human Genetics, University of Oxford, U.K; 2Department of Statistics, Oxford Centre for Gene Function, University of Oxford, U.K; 3MatTek Corporation, Ashland, MA, U.S.A. Presented at American Thoracic Society, San Diego, CA, May 20-25, (2005).
359. IDENTIFICATION OF INTELLIGENT BIOMARKERS OF EXPOSURE AND HARM IN THE RESPIRATORY EPITHELIAL TO TOBACCO SMOKE COMPONENTS.
BéruBé, K., Sexton, K., Jones, T. School of Biosciences, Cardiff University, Museum Avenue, Cardiff, CF10 3US, Wales, UK.
267. TUMOR NECROSIS FACTOR ALPHA (TNFα) INDUCTION OF THYMUS AND ACTIVATION REGULATED CHEMOKINE (TARC) EXPRESSION IS MEDIATED BY 15-LIPOXYGENASE 15-(LOX)-2 IN THE EPIAIRWAY™ HUMAN TRACHEAL/BRONCHIAL EPITHELIAL MODEL.
Jackson1, G.R., Last1, T.J., Klausner1, M., Kubilus1, J., Shappell2, S.B., Brash2, A.R. and Hayden1, P. 1MatTek Corporation, Ashland, MA USA, and 2Department of Pathology, Vanderbilt University School of Medicine, Nashville, TN USA. Presented at the American Thoracic Society Meeting, Atlanta, GA, May (2002).
256. EXPRESSION OF 15-LIPOXYGENASE-2 IN THE EPIAIRWAY™ IN VITRO HUMAN TRACHEAL/BRONCHIAL EPITHELIAL MODEL: REGULATION BY TNF-α AND INF-γ.
Hayden1, P.J., Jackson1, G.R., Last1, T.J., Klausner1, M., Kubilus1, J., Shappell2, S.B. 1MatTek Corp., Ashland, MA, 2Department of Pathology, Vanderbilt University School of Medicine, Nashville, TN. The Toxicologist, 66 (1-S), 100, Soc. of Toxicol. (Reston, VA), Abstract #486, (2002).
242. CHARACTERIZATION OF INFLAMMATORY MEDIATOR RELEASE FROM AN IN VITRO HUMAN TRACHEAL/BRONCHIAL EPITHELIAL TISSUE MODEL.
Hayden, P., Jackson, R., Last, T.J., Klausner, M. and Kubilus, J. MatTek Corp., Ashland, MA 01721. The Toxicologist, 60 (1), 425, Soc. of Toxicol. (Reston, VA), Abstract #2023, (2001).
225. CHARACTERIZATION OF INFLAMMATORY MEDIATOR RELEASE FROM AN IN VITRO HUMAN BRONCHIAL/TRACHEAL EPITHELIAL TISSUE MODEL.
Hayden, P., Jackson, R., Klausner, M., Kubilus, J. MatTek Corp., Ashland, MA. Presented at the Lovelace Respiratory Research Institute International Symposium, Santa Fe, NM, October 3, (2000).



www.MatTek.co.kr
|