The EpiVaginal ™ Tissue Model
주문은 최소 1개월 전에 하셔야합니다.
- Human vaginal-ectocervical tissues
- 3-dimensional, highly differentiated
- Contain normal human cells
- Serum-free medium available
- Highly reproducible
- Grown in Easily handled cell culture inserts
- Quantifiable, objective endpoints
- Ideal for toxicity studies of feminine hygiene, vaginal care, and microbicide products
- Useful for the study of HIV-1 and other sexually transmitted infections (STI)
- Cost effective alternative to animal and clinical testing
To facilitate the study of vaginal-ectocervical (VEC) toxicity, pathologies, and basic mucosal phenomena, MatTek has developed the EpiVaginal series of tissue models. EpiVaginal tissues are based on normal, human-derived VEC epithelial and dendritic cells (DC). Four types of EpiVaginal are offered:
1) VEC-100 (Figure 1): An epithelial tissue containing epithelial VEC cells,
2) VLC-100 (Figure 1): A patented epithelial tissue containing epithelial VEC and immuno-competent dendritic cells,
3) VEC-100-FT (Figure 2): A full thickness version of VEC-100 which includes VEC epithelial cells and a fibroblast-containing lamina propria, and
4) VLC-100-FT (Figure 2): A patented immuno-competent version of the VEC-100-FT which includes dendritic cells.
All VEC tissues are cultured on specially prepared cell culture inserts and are multilayered and highly differentiated. The tissues closely parallel native human tissues, thus providing a useful in vitro means to assess toxicity, sexually transmitted infections, and other basic vaginal phenomena.

Figure 1: H&E stained histological (formalin fixed) cross-sections of: A) VEC-100 and VLC-100 in vitro reconstructed epithelial tissue models containing normal human VEC cells (VEC-100) or VEC + DC cells (VLC-100), and B) vaginal explant tissue. Both in vitro and in vivo tissues show nucleated basal and suprabasal cell layers followed by layers in which nuclei are lost and cells are filled with glycogen. Click on photo to see larger image.

Figure 2: H&E stained histological (formalin fixed) cross-sections of full-thickness EpiVaginal tissues. The VEC-100-FT tissue consists of VEC epithelial cells cultured atop a lamina propria (LP)-like collagen matrix that contains fibroblasts. The VLC-100-FT consists of VEC epithelial and dendritic cells in the epithelial layers and contains both fibroblasts and dendritic cells in the LP. Click on photo to see larger image.
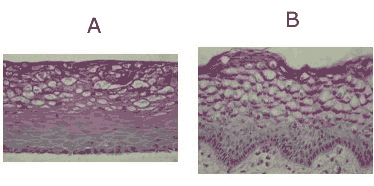
Figure 3: PAS Staining for Glycogen. Photo-micrographs of PAS reacted cross-sections of: A) reconstructed vaginal ectocervical tissue (VEC-100) and B) excised human vaginal tissue. Counter stain = hematoxylin. Glycogen shows up reddish-pink. In both tissues, the intensity of glycogen staining increases as the apical surface is approached. Note: In vivo, glycogen is released by the epithelial cells and fermented to lactic acid by lactobacilli (i.e. Doderlein's bacilli) that are part of the normal vaginal flora, accounting for the mildly acidic pH of vaginal fluid. Click on photo to see larger image.

Figure 4: Formalin fixed cross-sections of the VEC-100 tissue immuno-stained for cytokeratins, CK13, CK14, & CK18. Basal cells are stained by CK14 and supra-basal cells by CK13; no cells are stained by CK18. Click on photo to see larger image.
The EpiVaginal tissue models exhibit in vivo-like morphological and growth characteristics which are uniform and highly reproducible. EpiVaginal is a multilayered tissue consisting of an organized basal layer and multiple non-cornified layers analogous to native human vaginal-ectocervical tissue (Figures 1-3). The tissue expresses cytokeratin K14 in the basal and supra basal layers and cytokeratin K13 in the suprabasal tissue layers (Figure 4).
Various industrial and toxicology laboratories are actively seeking alternatives to expensive clinical or whole animal testing. The protocols for using EpiVaginal are clear and straightforward. Feminine hygiene, personal care, and pharmaceutical companies have initiated in vitro toxicology testing to evaluate their raw materials and final product formulations.
Table 1: EpiVaginal ET-50 ranges for various feminine hygiene products
|
Material Type |
ET-50 Range |
|
Triton X-100 (1%)
|
0.75-1.75 hr
|
|
Feminine washes (3 tested)
|
1-3 hrs
|
|
Spermicides (4 tested)
|
3-7 hrs
|
|
Anti-itch creams (4 tested)
|
6-18 hrs
|
|
Lubricant (2 tested)
|
>24 hrs
|
|
Antifungal products (3 tested)
|
>24 hrs
|
|
Douche (2 tested)
|
>24 hrs
|
Method: Duplicate VEC-100 tissues were treated with 83 uL of various feminine hygiene products for a range of exposure times. Following exposure, the product was washed from the VEC-100 tissue and the viability of the VEC-100 tissue was determined using the MTT assay. The exposure time that reduced the tissue viability to 50% (ET-50) was determined by interpolating between exposure times which bracketed 50% viability. A lower ET-50 indicates a more aggressive, more irritating product.
Figure 5 shows the effects of formulations containing the common spermicide active ingredient, nonoxynol-9 (N9), on the VEC-100-FT tissue. Tissue viability (MTT assay) (Figure 5A) and cytokine release (IL-1ß) (Figure 5B) were measured. As shown therein, with increasing N9 concentration, the tissue viability decreases and the IL-1ß release increases. Such assays can be used to predict toxicity of vaginal care products, microbicides, and other chemical agents.
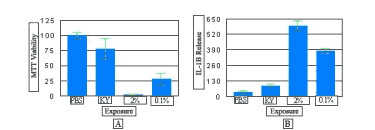
Figure 5: Viability (A) and cytokine release (B) of full thickness vaginal-ectocervical (VEC-100-FT) tissue model following exposure (18 hr) to different formulations containing nonoxynol-9 (N9): a) PBS control, b) KY Jelly (KY, 0% N9), c) KY N9 (2%), and d) N9 (0.1%). As viability of the tissue decreases, the IL-1ß release increases. Click on photo to see larger image.
Straight forward protocols are available for harvesting RNA to analyze gene expression or for measuring cytokines released into the culture medium (analyzed using ELISA assays). Companies and researchers utilize antibiotic/antifungal free EpiVaginal tissue (VEC-100-AFAB) to grow various opportunistic infections and pathogenic microbes in order to study their effects on the vaginal tissues. In addition, the VLC models are infectable with HIV-1 (Figure 6) and can be used to study HIV infection, transmission, and microbicides intended to prevent heterosexual passage of HIV. Finally, the tissue is amply suited to study a broad variety of pathogens that invade the vaginal-ectocervical environment along with prophylactic remedies there to.
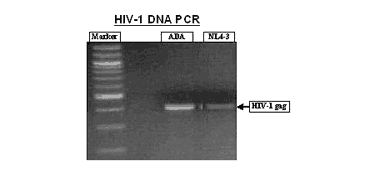
Figure 6: HIV-1 infection of VLC-100 tissue following topical exposure (24hr) to 60,000 CPM of HIV-1 ADA or NL4-3 virus. After exposure, tissues were washed 3X and then cultured for an additional 24 hr. Cellular DNA was then extracted and HIV-1 transcripts were detected using HIV-1 gag specific primer pairs. Click on photo to see larger image.
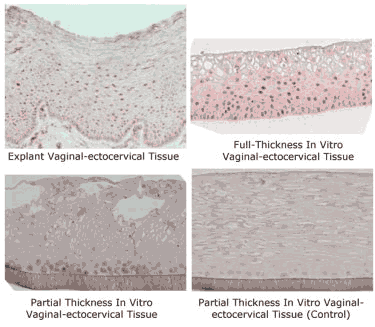
Figure 7: Micrographs showing nuclear staining of estradiol receptor (red) on: 1) Explant vaginal-ectocervical tissue (positive control), 2) Full-Thickness vaginal-ectocervical tissue model (VEC-FT), 3) partial-thickness vaginal-ectocervical (VEC) tissue, and 4) VEC tissue as negative control (without antibody). Formalin fixed tissues were labeled with rabbit anti-estrogen receptor (1:20 dilution) (Zymed Laboratories, Inc, South San Francisco, CA), incubated for 2 hr at room temperature, washed, and color was developed using alkaline phosphatase with Fast Red as substrate. Click on photo to see larger image.
Microbicides Testing - EpiVaginal™
Infection through the vaginal-ectocervical (VEC) tissue is believed to be the main route for the heterosexual transmission of the human immunodeficiency virus (HIV) in women.
Recently, a tissue culture-based model of the VEC (EpiVaginal) has been developed. Normal, human VEC epithelial and dendritic cell co-cultures were used to form a three-dimensional tissue using specially formulated medium. The in vitro engineered tissue reproduces many of the histological and ultrastructural features including basal, parabasal, glycogenated intermediate, and the superficial cell layers.
Preliminary experiments showed the use of this tissue model and the MTT tissue viability assay for predicting VEC irritation of microbicides*. Different concentrations of Nonoxynol-9 (N-9), carrageenin-I, and methyl cellulose were dosed topically and the viability of the VEC tissue was determine by MTT.
Following 24 hr exposure to N-9 (0.1%) tissue viability was reduced to 51%. In contrast, carrageenin-I (20%) reduces viability to 77% and no effect was observed for methyl cellulose (up to 20%). H & E staining showed irritation of epithelial lining following N-9 treatment greater than or equal to 0.1%. Experiments also showed that HIV virions do not pass freely through the tissue but they infect cells in the reconstructed VEC tissue model containing dendritic cells.
In conclusion, the tissue model is likely to serve as a useful tool to screen new or existing microbicide formulations for vaginal toxicity and microbicidal efficacy.
====================
Excerpted from, "HUMAN VAGINAL-ECTOCERVICAL TISSUE MODEL FOR MICROBICIDE IRRITATION STUDIES" (MatTek Corp. Technical Reference TR-320) - Presented at "Microbicides 2004", London, England, March 28-31, (2004).