The EpiAirway™ Tissue Model
최소 1개월전에는 주문을 하여야 합니다.
Features
EpiAirwayTM Tissue Model
- Normal Human 3D (NHu-3D) Model of Rispiratory Tract Tissue
- Produced From Normal Tracheal/Bronchial Epithelial Cells
- Long-Term Lifespan for Chronic Exposure Studies
- Asthmatic, Smoker, COPD and Goblet Cell Hyperplasia Available
- Ciliated, Human Bronchiole-Like Structure
- Contains Mucin Producing Goblet Cells
- Tight Junctions/Electrogenic Tissue
- Available in Individual Inserts, 24-Well HTS and 96-Well HTS Formats
- Compatible with VITROCELL Apparatus
- Drug Delivery/Discovery Tool
- Ideal for Gene Expression Analysis and RNAi/siRNA-Based Therapeutic Drug Screening
General MatTek Tissue Features
- Unsurpassed Long-Term Tissue Reproducibility
- 3-Dimensional, Highly Differentiated Tissue
- Metabolically, Mitotically Active
- Produced from Normal (Non-Transformed) Human Cells - Ideal for Genomics Studies
- Produced in Easily Handled Cell Culture Inserts
- Grown in Completely Serum-Free Media System
- Quantifiable, Objective Test Endpoints
- Cost Effective Alternative to Animal and Human Clinical Testing
Applications
Major EpiAirway Applications ---> 바로가기
Airway Models of Asthma and COPD ---> 바로가기
Data Sheet
EpiAirwayTM Tissue Model
MatTek's EpiAirway™ System consists of normal, human-derived tracheal/bronchial epithelial cells (TBE) which have been cultured to form a multilayered, highly differentiated model which closely resembles the epithelial tissue of the respiratory tract. Histological cross-sections of both the in vitro tissue and a normal human bronchiole reveal a pseudostratified epithelial structure (Figure 1).
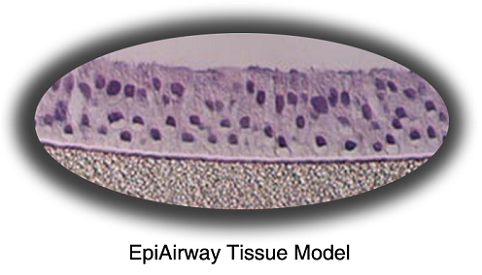
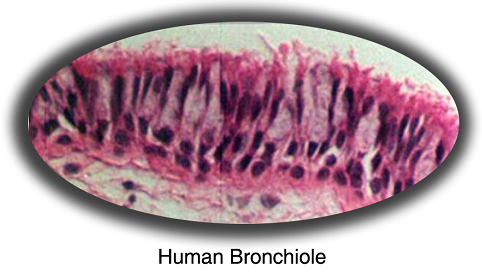
Figure 1. Histology - EpiAirwayTM and Normal Human Bronchiole Formalin Fixed, Paraffin Embedded and H&E Stained (100x).
Transmission electron microscopy shows numerous microvilli and cilia on the apical surface of the cultures and confirm the presence of tight junctions (Figure 2). Transepithelial electrical resistance of the tissue is similar to in vivo tissue. Mucins are secreted at the apical surface (Figure 3).
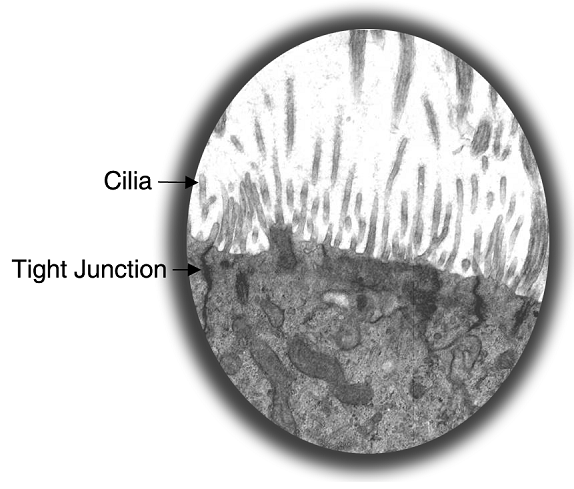
Figure 2. TEM Micrograph - EpiAirwayTM showing cilia on the apical surface and a tight junction between the cells.
The EpiAirway cultures are grown on cell culture inserts at the air-liquid interface, allowing for gas phase exposure of volatile materials for airway inflammation and irritancy studies. This convenient format also allows the facile measurement of transepithelial permeability for inhaled drug delivery studies. The tissues can also be used to investigate mechanisms of bacterial infection of the respiratory tract (See Figure 4). These and other studies involving asthma, cytokine responses, or various airway disorders can be performed using the EpiAirway tissue.
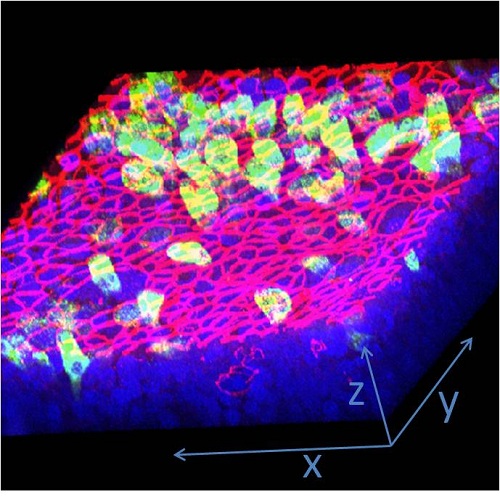
Figure 3. 3D Confocal Image - EpiAirwayTM showing mucin (Muc5AC, green) staining in cells at the apical surface and tight junction (ZO-1, red) staining between the apical cells. Nuclei have been counterstained (DAPI, blue).
Various pharmaceutical laboratories are actively seeking alternatives to expensive and time consuming pre-clinical animal testing. Many companies have initiated EpiAirway testing to assess the ability of candidate compounds to modulate specific respiratory properties of interest. A growing body of data demonstrates that EpiAirway provides a cost-effective means of assessing various respiratory tract issues while avoiding species extrapolation and the use of laboratory animals.
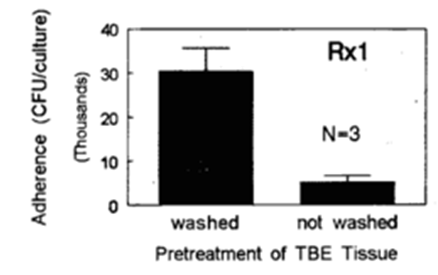
Figure 4: Bacterial adherance- The effect of mucin secretion on adherance of S. pneumoniae to EpiAirway™ tissues for nonencapsulated strain Rx1. Bacteria/epithelia cell ratio was 15:1. EpiAirway™ tissues were washed 3X with HEPES buffered saline to remove surface secreted mucin (or left unwashed) prior to exposure to bacteria. (Data graciously provided by MedImmune, Inc.).
 |
|
|
| |
|
Cilia------> |
 |
|
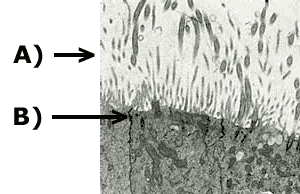
A) |
|
|
| |
|
|
Ciliated apical surface -> |

|
|
B) |
|
Collagen matrix at
basolateral surface --> |
| |
|
|
Cilia------> |
|
|
C) |
|
Surrounding lung
tissue --> |
| |
|
|
Figure 6: H&E stained cross-sections of: A) EpiAirway tissue (10X objective), B) EpiAirway tissue (40X objective), and C) normal human bronchiole (10X objective) showing pseudo-stratified differentiated mucociliary phenotype. | |
|
Figure 7: TEM micrograph of EpiAirway tissue showing: A) cilia on apical tissue surface and B) tight junction between cells.
|
|
|
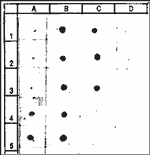 |
Figure 8: Mucin Analysis - Immunoblots for washings of apical surface of EpiAirway™ and control skin (EpiDerm™) tissues.
Key: PB = phosphate buffered saline; TBS Tris buffered saline; Mucin = Human mucin standard; TBE #1 - #7: different media formulations. TBE #2 final formulation. Based on a comparison of dots A5, B1 and B3, the mucin level in TBE #2 = 8 mg/ml (Note: dot B3 has been diluted to 10%).
|
| |
A |
B |
C |
D |
|
1 |
PBS |
Mucin
1 mg/ml |
TBE #5
1:10 |
EpiDerm
undiluted |
|
2 |
TBS |
TBE #1,
1:10 |
TBE #6
1:10 |
EpiDerm
1:10 |
|
3 |
Mucin
0.01 mg/ml |
TBE #2,
1:10 |
TBE #7
1:10 |
EpiDerm
1:10 |
|
4 |
Mucin
0.033
mg/ml |
TBE #3
1:10 |
EpiDerm
undiluted |
EpiDerm
1:10 |
|
5 |
Mucin
0.1 mg/ml |
TBE #4,
1:10 |
EpiDerm
undiluted |
|



www.MatTek.co.kr