|
Figure 4. Expression of N-Cadherin Adhesion Molecule in MLNM-FT-A375 Full Thickness Melanoma Skin Model. N-Cadherin antibody staining, 40X. Note increased level of N-Cadherin expression with time in culture.
Applications
Tumor Invasion

703. FISETIN INHIBITS HUMAN MELANOMA CELL INVASION THROUGH PROMOTION OF MESENCHYMAL TO EPITHELIAL TRANSITION AND BY TARGETING MAPK AND NFкB SIGNALING PATHWAY.
Pal, H.C., Sharma, S., Elmets, C.A. and Afaq, F. Department of Dermatology, University of Alabama at Birmingham, Birmingham, AL, USA. Presented at 2012 Society of Investigative Dermatology Meeting, May 2012.
455. ROSCOVITINE INHIBITS DIFFERENTIATION AND INVASION IN A THREE-DIMENSIONAL SKIN RECONSTRUCTION MODEL OF METASTATIC MELANOMA.
Mohapatra1, S., Coppola1, D., Riker2, A.I., and Pledger1, W.J. 1Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center and Research Institute and the University of South Florida Medical Center, Tampa, Florida, 2University of South Alabama-Mitchell Cancer Institute, Mobile, Alabama. Mol Cancer Res; 5, (2), 145–51, (2007).
Anti-Melanoma Drug Screening

728. THE ROLE OF MELANOMA TUMOR-DERIVED NITRIC OXIDE IN THE TUMOR INFLAMMATORY MICROENVIRONMENT: ITS IMPACT ON THE CHEMOKINE EXPRESSION PROFILE, INCLUDING SUPPRESSION OF CXCL10.
Tanese1,2, K., Grimm1, E.A. and Ekmekcioglu1, S. 1Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX. 2Department of Dermatology, School of Medicine, Keio University, Tokyo, Japan. Int. J. Cancer, 131, 891–901 (2012).
703. FISETIN INHIBITS HUMAN MELANOMA CELL INVASION THROUGH PROMOTION OF MESENCHYMAL TO EPITHELIAL TRANSITION AND BY TARGETING MAPK AND NFкB SIGNALING PATHWAY.
Pal, H.C., Sharma, S., Elmets, C.A. and Afaq, F. Department of Dermatology, University of Alabama at Birmingham, Birmingham, AL, USA. Presented at 2012 Society of Investigative Dermatology Meeting, May 2012.
609. THE SKIN CANCER CHEMOTHERAPEUTIC AGENT INGENOL-3-ANGELATE (PEP005) IS A SUBSTRATE FOR THE EPIDERMAL MULTIDRUG TRANSPORTER (ABCB1) AND TARGETS TUMOR VASCULATURE.
Li1, L., Shukla2, S., Lee1, A., Garfield3, S.H., Maloney4, D.J., Ambudkar2, S.V. and Yuspa1, S.H. 1Laboratory of Cancer Biology and Genetics, 2Laboratory of Cell Biology, and 3Confocal Core Facility, Center for Cancer Research, National Cancer Institute and 4NIH Chemical Genomics Center, National Human Genome Research Institute, NIH, Bethesda, Maryland Cancer Res., 70(11), 4509–19, 2010.
525. UPREGULATION OF SOX9 INHIBITS THE GROWTH OF HUMAN AND MOUSE MELANOMAS AND RESTORES THEIR SENSITIVITY TO RETINOIC ACID.
Passeron, T., Valencia, J.C., Namiki, T., Vieira, W.D., Passeron, H., Miyamura, Y., and Hearing, V.J. Laboratory of Cell Biology, National Cancer Institute, NIH, Bethesda, Maryland, USA. J. Clin. Invest., 119, 954-963 (2009).
498. A SMALL-MOLECULE E2F INHIBITOR BLOCKS GROWTH IN A MELANOMA CULTURE MODEL.
Ma1, Y., Kurtyka1, C.A., Boyapalle1, S., Sung2, S-S. Lawrence2, H., Guida2, W., and Cress1, W.D. 1Molecular Oncology Program and 2High Throughput Screening and Chemistry Core Facility, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida. Cancer Res., 68 (15):6292–9 (2008).
Melanoma Skin Model (MLNM-FT-A375)
Technical Specifications
I. Ordering
- New Orders: MatTek Scientists will consult on your application at no additional charge. It is STRONGLY RECOMMENDED that you have this consultation BEFORE placing your first order to help ensure that all media and accessories needed to perform your application are included in the initial order.
- ALL Tissue Orders: Please allow 3-4 weeks from order date to delivery of tissues. Please contact MatTek Customer Service for additional details.
II. Cells
- Type: Human melanoma cells (A375); Normal human epidermal keratinocytes (NHEK); Normal human dermal fibroblasts (NHDF).
- Derived from: Malignant melanoma cell line (A375); Neonatal-foreskin tissue (NHEK); Neonatal skin (NHDF);
- Alternatives: Human malignant melanoma cells SK-Mel-28.
- Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma.
III. Medium
- Base medium: MCDB 153 Basal Medium.
- Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation.
- Serum: None.
- Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
- Anti-fungal agent: Amphotericin B 0.25 µg/ml.
- pH Indicator: Phenol red (0.0012 g/l).
- Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary).
- Assay/Maintenance medium: MLNM-FT-MM is utilized for assays as well as long term maintenance of the MLNM-FT-A375 tissues.
IV. Tissue
- Kit: Full thickness melanoma skin construct (MLNM-FT-A375 kit) consists of 24 tissues. (Tissue "kits" contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
- Substrate: Single well tissue culture plate inserts are used (e.g. Millicell CM single well tissue culture plate inserts, pore size = 0.4 µm, surface area = 0.6 cm2).
- Culture: Initially submerged and then at the air liquid interface.
- Histology: Epithelium: 8-12 epithelial cell layers plus stratum corneum (basal, spinous, and granular layers); Dermis: Collagen gel containing fibroblasts. At early stage melanoma cells form nodes at dermal/epidermal junction. At later stages melanomas progress to RGP (radial growth phase), VGP (vertical growth phase) and consecutively invade dermal component.
- Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
- Shipment: Tissues are shipped at 4°C on medium-supplemented, agarose gels in 6-well plates.
- Shipment day: Monday. Shipment on Thursday also possible upon special request.
- Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
- Shelf life: Including time in transit, tissues may be stored at 4°C for up to 3 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
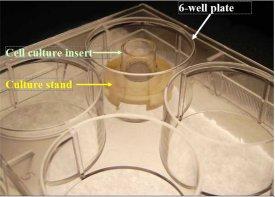
- Length of experiments: Cultures can be continued for up to 4 weeks for melanoma development and progression with good retention of normal epidermal morphology. Cultures must be fed every other day using MatTek culture stands (MEL-STND; see photo below) along with 5.0 ml of MLNM-FT-MM.
Alternative tissues:
MLNM-FT-A375 (Day 6): Early stage MLNM-FT-A375 tissue. Tissue has not been cultured at the Air-Liquid Interface. Customer receives required media (MLNM-FT-GM) to continue cultures and produce MLNM-FT-A375. Discussion with a MatTek technical representative is required prior to ordering due to proprietary nature of this product. Designed for study of early stages of melanoma development in which experimental design dictates use of early stage tissue.
MLNM-FT-EXP: Same as MLMN-FT-A375 except MatTek replaces A375 cells with customer-supplied melanoma cells.
 |
|
MEL-STND Accessory For Extended Culture Periods: Shown: 6-well plate with cell culture insert atop a culture stand (Part #MEL-STND). For experiments extending beyond 24 hours, tissues need to be fed with 5.0 ml every other day. Use of the culture stand allows feeding with 5.0 ml from the basolateral side of the tissue. Use of culture stands is necessary in order to maintain normal tissue morphology. Click on photo to see larger image. |
V. Quality Control and Sterility
- Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
- End-use testing: Tissues from the production lot are retained, grown for additional 7 days, fixed for histology, and analyzed.
- Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
- Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
- Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances sterility failures will be notified within 8 days of shipment).


|