Phototoxicity ( EpiDerm )
The Model
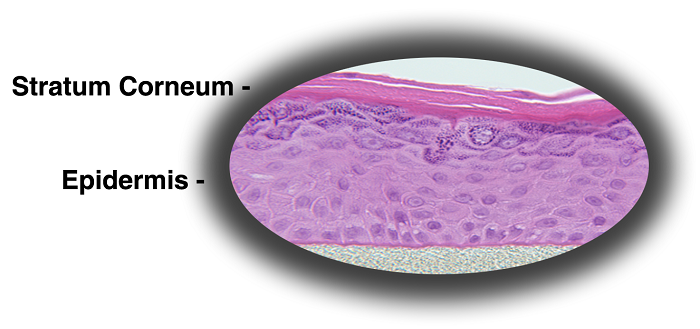
MatTek's patented EpiDerm™ System consists of normal, human-derived epidermal keratinocytes (NHEK) which have been cultured to form a multilayered, highly differentiated model of the human epidermis. These "ready-to-use" tissues, also known generically as Normal Human-3D (NHu-3D), are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro skin technology. Ultrastructurally, the EpiDerm Skin Model closely parallels human skin, thus providing a useful in vitro means to assess dermal irritancy and toxicology. For more information on the EpiDerm model, click here.
The Method
프로토콜 요청은 이곳으로.
-
Transfer tissues from agarose to assay medium
-
Incubate (37 ±1°C, 5 ±1% CO2, 95% RH) for 1h
- Transfer tissues to fresh assay medium
-
Set 1 - Dose 2 tissues each with 1 of 5 concentrations of TS or 1 VC
- Set 2 - Dose 2 tissues each with 1 of 5 concentrations of TS or 1 VC (identical to step 4.)
-
Expose tissues overnight (18-24 hrs) (37 ±1°C, 5 ±1% CO2, 95% RH)
- Irradiate Set 1 for 60 min with 1.7 mW/cm2 (6 J/cm2) at room temperature. Keep Set 2 in the dark.
-
Stop exposure and wash tissues with DPBS
- Transfer tissues to fresh assay medium
-
Incubate (37 ±1°C, 5 ±1% CO2, 95% RH) overnight (18 - 24 hrs)
-
Blot tissues and perform MTT assay
-
Read OD in a plate spectrophotometer at 550-570nm
The Endpoints
MTT Tissue Viability Assay

Technical References

588. PHOTOTOXICITY OF BERGAMOT OIL ASSESSED BY IN VITRO TECHNIQUES IN COMBINATION WITH HUMAN PATCH TESTS. Kjlova1, K., Jirova1, D., Bendova1, H., Kandarova2, H., Weidenhoffer1, Z., Kolarova3, H., Liebsch2, M. 1National Reference Center for Cosmetics, National Institute of Public Health, Srobarova 48, 100 42 Prague 10, Czech Republic. 2Federal Institute for Risk Assessment (BfR), ZEBET at the BfR, Berlin, Germany. 3Medical Faculty of Palacky University, Olomouc, Czech Republic. Toxicology in Vitro, 21, 1298–1303 (2007).
531. ALTERNATIVE METHODS FOR PHOTOTOXICITY TEST USING RECONSTRUCTED HUMAN SKIN MODEL. Sohn, S., Ju3, J.H., Son1, K.H., Lee3, J.P., Kim, J., Lim, C.H., Hong, S.K., Kwon, T.R., Chang, M., Kim2, D.S., Yoon3, H.S., Park2, K.L. Toxicological Evaluation and Research Department, 1Pharmaceuticals and Medical Devices Research Department, 2Center for Drug Development Assistance, National Institute of Food and Drug Safety Evaluation, 3KFDA. Altex, Vol 26, Spec. Issue, p. 136 (2009).
441. COMPARISON OF IN VITRO PHOTOTOXICITY TEST METHODS: 3T3 NRU PT VS. ENHANCED PHOTOTOXICITY SCREENING ASSAY IN RECONSTITUTED SKIN (EPARS). Pratt, L., Kirk, C., Reeder, M., DeGeorge, G. MB Research Laboratories, Spinnerstown, PA. Society of Toxicology 46th Annual Meeting, Charlotte, NC, (2007)., Poster 441. The Toxicologist, 96, 1, 315 (2007).
412. IN VITRO SCREEN FOR PHOTOTOXICITY OPTIMIZED DRUG DEVELOPMENT USING A HIGHLY DIFFERENTIATED SKIN MODEL. Klausner, M., Neal, P., Kubilus, J. MatTek Corporation, Ashland, MA. Presented at the AAPS 2006 Biotechnology Conference, Boston, MA., June (2006).
316. AN ASSESSMENT OF THE PHOTOTOXIC HAZARD OF A PERSONAL PRODUCT INGREDIENT USING IN VITRO ASSAYS. Jones, P.A., King, A.V., Earl, L.K., Lawrence, R.S. Unilever Colworth, Bedfordshire, UK. Toxicology in Vitro, 17, 471-480, (2003).








www.MatTek.co.kr